This study aimed to assess the impacts of addition of CaCO3 as a coadjuvant in oil yield from Avocado Pear seed in a soxhlet extraction apparatus with ethanol and n-hexane as solvents. Response surface methodology (RSM) was employed to optimize parameters influencing oil yield during solvent extraction. A Box Behnken design with three factors: concentration of CaCO3 (1, 1.5, 2%), extraction time (90, 145, 180 min), and extraction temperature (25, 35, 45°C) was utilized for the optimization process. The linear model provided the most accurate fit to the experimental data with determination coefficient (R2) value of 0.8273. The optimized yield of avocado oil was 4.2343% for n-hexane and 6.8156% for ethanol. The fatty acids present in the oil were determined using gas chromatography, and oleic acid was the dominant fatty acid. The values of main physiochemical properties evaluated were: Light Honey brown, Honey brown – colour; Fruity odour, Fruity odour – odour; 150.0114mgKOH/g, 67.32mgKOH/g – saponification value; 1.1809506%, 1.864302% – free fatty acid; 44.892mgI/g, 65.736mgI/g – iodine value; 1.95%, 1.17% – Moisture content; 5.07, 5.04 – pH and 0.291 meq/kg, 0.558 meq/kg – peroxide value for n-hexane and ethanol solvents, respectively. The oil high saponification value and low iodine value makes it applicable in soap making industries, as lubricant in industrial machine and as a stabilizer in other industrial process.
| Published in | American Journal of Applied and Industrial Chemistry (Volume 8, Issue 2) |
| DOI | 10.11648/j.ajaic.20250802.12 |
| Page(s) | 41-52 |
| Creative Commons |
This is an Open Access article, distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium or format, provided the original work is properly cited. |
| Copyright |
Copyright © The Author(s), 2025. Published by Science Publishing Group |
Avocado Pear Seed, Extraction, CaCO3, Response Surface Methodology, Optimization
Variables | Coded values | ||
|---|---|---|---|
-1 | 0 | 1 | |
Concentration of CaCO3, X1 (% w/w) | 1 | 1.5 | 2 |
Extraction time, X2 (mins) | 90 | 135 | 180 |
Extraction temperature, X3 (°C) | 25 | 35 | 45 |
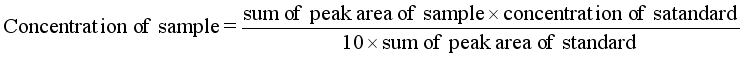 (2)
(2) 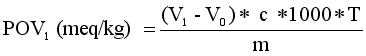 (6)
(6) Run | X1: Concentration (%) | X2: Extraction time (min) | X3: Temperature (°C) | Res 1: Oil yield 1 - Ethanol (%) | Res 2: Oil yield 2 - N-hexane (%) |
|---|---|---|---|---|---|
1 | 2 | 135 | 45 | 9.205 | 4.170 |
2 | 1.5 | 135 | 35 | 7.250 | 3.955 |
3 | 2 | 135 | 25 | 6.005 | 4.050 |
4 | 1 | 180 | 35 | 9.380 | 5.740 |
5 | 1 | 90 | 35 | 6.300 | 3.400 |
6 | 1.5 | 180 | 45 | 9.575 | 6.195 |
7 | 1.5 | 135 | 35 | 7.295 | 3.540 |
8 | 2 | 90 | 35 | 6.250 | 3.135 |
9 | 1.5 | 90 | 45 | 6.590 | 3.255 |
10 | 1.5 | 135 | 35 | 7.550 | 4.145 |
11 | 1.5 | 90 | 25 | 5.990 | 2.655 |
12 | 1.5 | 180 | 25 | 7.490 | 5.175 |
13 | 1.5 | 135 | 35 | 7.495 | 4.120 |
14 | 1 | 135 | 45 | 8.535 | 3.555 |
15 | 2 | 180 | 35 | 8.015 | 5.625 |
16 | 1.5 | 135 | 35 | 7.900 | 4.320 |
17 | 1 | 135 | 25 | 5.725 | 3.350 |
Solvent: Ethanol | ||||||
|---|---|---|---|---|---|---|
Source | Sum of squares | Df | Mean square | F-value | p-value | Remark |
Model | 20.36 | 3 | 6.79 | 25.68 | < 0.0001 | Significant |
X1: Conc. of CaCO3 | 0.027 | 1 | 0.027 | 0.1023 | 0.7542 | |
X2: Extraction time | 10.88 | 1 | 10.88 | 41.18 | < 0.0001 | |
X3: Temperature | 9.45 | 1 | 9.45 | 35.76 | < 0.0001 | |
Residual | 3.44 | 13 | 0.2643 | |||
Lack of Fit | 3.17 | 9 | 0.352 | 5.27 | 0.0622 | Not significant |
Pure Error | 0.267 | 4 | 0.0668 | |||
Cor Total | 23.79 | 16 | ||||
Solvent: N-hexane | ||||||
Model | 13.82 | 3 | 4.61 | 27.34 | < 0.0001 | Significant |
X1: Conc. of CaCO3 | 0.1093 | 1 | 0.1093 | 0.6486 | 0.4351 | |
X2: Extraction time | 13.24 | 1 | 13.24 | 78.56 | < 0.0001 | |
X3: Temperature | 0.4729 | 1 | 0.4729 | 2.81 | 0.1177 | |
Residual | 2.19 | 13 | 0.1685 | |||
Lack of Fit | 1.84 | 9 | 0.2045 | 2.34 | 0.2149 | Not significant |
Pure Error | 0.3502 | 4 | 0.0875 | |||
Cor Total | 16.01 | 16 | ||||
Parameters | Solvents | |
|---|---|---|
Ethanol | N-hexane | |
Standard deviation | 0.5141 | 0.4105 |
Mean | 7.44 | 4.14 |
Coefficient of Variance | 6.91 | 9.91 |
0.8556 | 0.8632 | |
Adjusted | 0.8223 | 0.8316 |
Predicted | 0.7092 | 0.7393 |
Adeq. Precision | 18.0715 | 15.3625 |
Solvent: Ethanol | Solvent: N-hexane | ||||||
|---|---|---|---|---|---|---|---|
In terms of Coded Equation | In terms of Actual Equation | In terms of Coded Equation | In terms of Actual Equation | ||||
Avocado oil yield | = | Avocado oil yield | = | Avocado oil yield | = | Avocado oil yield | = |
7.44 | 0.31568 | 4.14 | -0.92 | ||||
-0.0581 | X1 | -0.11625 | CaCO3 conc | 0.1169 | X1 | 0.23375 | CaCO3 conc |
1.1700 | X2 | 0.025917 | Extraction time | 1.2863 | X2 | 0.02858 | Extraction time |
1.0900 | X3 | 0.108687 | Extraction temperature | 0.2431 | X3 | 0.043123 | Extraction temperature |
Solvent: Ethanol | ||||
|---|---|---|---|---|
Component | Retention | Area | Height | External units (ppm) |
Solvent | 0.466 | 88679.8875 | 5637.211 | 0.0000 |
Palmitic acid | 5.066 | 1609.9960 | 88.691 | 188.7495 |
Palmitoleic acid | 6.200 | 409.4220 | 31.565 | 40.9422 |
Stearic acid | 6.566 | 1033.9615 | 52.701 | 0.0000 |
Oleic acid | 7.800 | 10207.3055 | 774.317 | 0.0000 |
Linoleic acid | 9.583 | 1461.690 | 54.628 | 0.0000 |
Solvent: N-hexane | ||||
Solvent | 0.466 | 87811.0310 | 5631.974 | 0.0000 |
Palmitic acid | 5.066 | 784.7640 | 73.594 | 85.5955 |
Palmitoleic acid | 6.200 | 291.8030 | 29.850 | 29.1803 |
Stearic acid | 6.566 | 821.3770 | 47.648 | 0.0000 |
Oleic acid | 7.800 | 8946.4860 | 766.036 | 0.0000 |
Linoleic acid | 9.466 | 780.5440 | 42.091 | 0.0000 |
Physiochemical Parameters | N-hexane Observation/values | Ethanol Observation/values |
|---|---|---|
Colour | Light Honey brown | Honey brown |
Odour | Fruity odour | Fruity odour |
Moisture content | 1.95% | 1.17% |
Iodine value | 44.892mgI/g | 65.736mgI/g |
Saponification | 150.0114mgKOH/g | 67.32mgKOH/g |
pH | 5.07 | 5.04 |
Peroxide value | 0.291 meq/kg | 0.558 meq/kg |
Free fatty acid value | 1.1809506% | 1.864302% |
| [1] |
Ekpa, O. D. and Isaac, I. O. (2013). Fatty acid composition of melon (Colocynthis vulgaris Shrad) seed oil and its application in synthesis and evaluation of alkyd resins. International Organization of Scientific Research Journal of Applied Chemistry, 4(4), 30 – 41.
https://www.iosrjournals.org/iosr-jac/papers/vol4-issue4/F0443041.pdf |
| [2] | Ying, Q., Rudzińska, M., Grygier, A. and Przybylski, R. (2020). Determination of triacylglycerols by HTGC-FID as a sensitive tool for the identification of rapeseed and olive oil adulteration. Molecules, 25(17), 3881 – 3891. |
| [3] | Gnanaprakasam A., Sivakumar V. M., Surendhar A., Thirumarimurugan M., Kannadasan T. (2013). Recent strategy of biodiesel production from waste cooking oil and process influencing parameters: a review. Energy, 7, 1 – 10. |
| [4] | Gharby, S., Guillaume, D., Elibrahimi, M. and Charrouf, Z. (2021). Physico-chemical properties and sensory analysis of deodorized argan oil. ACS Food Science and Technology, 1(2), 275 – 281. |
| [5] | Araújo, R. G., Rodriguez-Jasso, R. M., Ruiz, H. A., Pintado, C. N. and Aguilar, M. E. (2018). Avocado by-products: Nutritional and functional properties. Trends in Food Science and Technology, 80, 51 – 60. |
| [6] | Wang, W., Bostic, T. R. and Gu, L. (2010). Antioxidant capacities, procyanidins and pigments in avocados of different strains and cultivars. Food Chemistry, 122(4), 1193 – 1198. |
| [7] | López, N. J., Domínguez-Avila, J. A., Yahia, E. M., Belmonte-Herrera, B. H., Wall-Medrano, A., Montalvo-González, E., González-Aguilar, G. A. (2020). Avocado fruit and by-products as potential sources of bioactive compounds. Food Research International. 138, 109774. |
| [8] | Fajriyati, M., Meta, M., Amran, L. and Zainal, Z. (2017. Optimization of mango seed kernel oil extraction using response surface methodology. Oilseeds & fats, Crops and Lipids, 24(5); D503; 1 – 7. |
| [9] | Shende, D. and Sidhu, G. K. (2016). Response surface methodology to optimize enzyme-assisted aqueous extraction of maize germ oil. Journal of Food Science and Technology, 53, 3282–3295. |
| [10] |
Food and Drug Administration (FDA), Database of Select Committee on GRAS Substances Reviews. Report No. 26, CFR Section 184.1191.
http://www.accessdata.fda.gov/scripts/fdcc/?set¼SCOGS (accessed date April 2015). |
| [11] | Akinoso, R., Aboaba, S. A. and Olajide, W. O. (2011). Optimization of roasting temperature and time during oil extraction from orange (Citrus sinensis) seeds: A response surface methodology approach. African Journal Food Agriculture Nutrition Development, 11(6), 5300 – 5317. |
| [12] | Faiznur, M. F., Khairiah, A. K. and Mashitah, M. D. (2016). Ultrasonic-assisted extraction of oil from Calophyllum inophllum seeds: Optimization of process parameters. Physical Sciences, 27(2), 103 – 121. |
| [13] | Yenge, G. B., Kanawade, V. L., Nimbalkar, C. A., Kenghe, R. N., Patil, A. P. and More, H. G. (2017). Optimization of Soxhlet extraction of garden cress oil by response surface methodology. Environmental Science and Chemistry, 5, 526 – 530. |
| [14] | Humairah, R., Annisa, D., Moulana, R., Maulida, A., Supardan, M. D., Syamsuddin, Y. and Satriana, S. (2024). Optimization of avocado oil solvent extraction assisted by CaCO3 as coadjuvant using Response Surface Methodology. Institute of Physics Conference Series 2023: Earth Environmental Science 1290(1): 012013. |
| [15] | Mgoma, S. T., Gutu, L., Basitere, M., Mshayisa, V. (2019). Effects of Different Extraction Methods and Process Conditions in the Yields of Avocado Oil. 16th South Africa International Conference on Agricultural, Chemical, Biological and Environmental Sciences (ACBES-19). Eminent Association of Pioneers (EAP), Johannesburg, 271– 275. |
| [16] | ASTM D7678-17 (2022). Standard Test Method for Total Petroleum Hydrocarbons (TPH) in Water and Wastewater by Gas Chromatography. West Conshohocken, USA. |
| [17] | American Oil Chemist’ Society (2017). Official method Cd 8b-90. Peroxide value, acetic acid, and oil isooctane method, 7th edition. Official Methods and Recommended Practices of the AOCS, USA. |
| [18] | AOAC, (2000). Official Methods of Analysis, Association of Official Analytical Chemists, 17th edition, Washington DC, USA. |
| [19] | AOCS Method cd 3-25 (1993). Official Methods of Analysis, Association of Official Analytical Chemists, Washington DC, USA. |
| [20] | AOCS Method 981.12 (1990). Official Methods of Analysis, Association of Official Analytical Chemists, Washington DC, USA. |
| [21] | AOAC Method 965.33 (1969) Official Methods of Analysis, Association of Official Analytical Chemists, Washington DC, USA. |
| [22] | AOCS Method Ca 5a-40 (1997). Official Methods of Analysis, Association of official Analytical Chemists, Washington DC, USA. |
| [23] | Squeo, G., Silletti, R., Summo, C., Paradiso, V. M., Pasqualone, A. and Caponio, F. (2016). Influence of calcium carbonate on extraction yield and quality of extra virgin oil from olive (Olea europaea L. cv. Coratina). Food Chemistry 209(1), 65 – 71. |
| [24] | Ginting, Z.; Nurhakim, L.; Hakim, L. (2020). Production of Avocado Oil from Gayo Avocado Pulp by Extraction Method Using N-Hexane Solvent for Application in Herbal Bath Soap. Unimal Journal of Chemical Technology, 9(1), 58 – 67. |
| [25] | Otaigbe, J., Oriji, O. and Ekerenam, G. (2016). Studies on the Paint Forming Properties of Avocado (Persea americana) and African Pear (Dacryodes edulis) Seed Oils. International Journal of Engineering Research and Application, 6(12), 8 – 15. |
| [26] | Arukwe, U., Amadi, B. A., Duru, M. K. C., Agomuo, E. N., Adindu, E. A., Odika, P. C. and Anudike, J. (2012). Chemical composition of Persea americana leaf, fruit and seed. International Journal of Recent Research and Applied Studies, 11(2), 346 – 349. |
| [27] | Musa, U., Aboje A. A., Mohammed, I. A., Aliyu M. A., Sadiq, M. M. and Olaibi, A. O. (2014). The Effect of Process Variables on the Transesterification of Refined Cottonseed Oil. Proceedings of the World Congress on Engineering, Vol I, (WCE 2014), July 2 - 4, 2014, London, U.K, 622 – 624. |
| [28] | Musa, U., Isah, A. G., Garba, M. U., Zelihatu, U. and Bello, A. U. (2015). Extraction of Chrysophyllum albidum Seed Oil: Optimization and Characterization, Chemical and Process Engineering Research, 30, 1 – 9. |
| [29] | Ikhuoria, E. U and Maliki, M. (2007). Characterization of avocado pear (Persea americana) and African pear (Dacryodes edulis) extracts, African Journal of Biotechnology, 6(7): 950 – 952. |
| [30] | Akintunde, A. M, Ajala, S. O, Betiku, E (2015). Optimization of Bauhinia monandra seed oil extraction via artificial neural network and response surface methodology: A potential biofuel candidate. Industrial Crops and Products, 67, 3 87 – 394. |
APA Style
Alao, A. I., Lawal, Z. A., Akinwumi, O. D. (2025). Extraction of Avocado Seed Oil with Ethanol and N-hexane Using CaCO3 as Coadjuvant: Optimization and Characterization. American Journal of Applied and Industrial Chemistry, 8(2), 41-52. https://doi.org/10.11648/j.ajaic.20250802.12
ACS Style
Alao, A. I.; Lawal, Z. A.; Akinwumi, O. D. Extraction of Avocado Seed Oil with Ethanol and N-hexane Using CaCO3 as Coadjuvant: Optimization and Characterization. Am. J. Appl. Ind. Chem. 2025, 8(2), 41-52. doi: 10.11648/j.ajaic.20250802.12
@article{10.11648/j.ajaic.20250802.12,
author = {Adeyinka Idowu Alao and Zainab Ayotomiwa Lawal and Odunayo Deborah Akinwumi},
title = {Extraction of Avocado Seed Oil with Ethanol and N-hexane Using CaCO3 as Coadjuvant: Optimization and Characterization
},
journal = {American Journal of Applied and Industrial Chemistry},
volume = {8},
number = {2},
pages = {41-52},
doi = {10.11648/j.ajaic.20250802.12},
url = {https://doi.org/10.11648/j.ajaic.20250802.12},
eprint = {https://article.sciencepublishinggroup.com/pdf/10.11648.j.ajaic.20250802.12},
abstract = {This study aimed to assess the impacts of addition of CaCO3 as a coadjuvant in oil yield from Avocado Pear seed in a soxhlet extraction apparatus with ethanol and n-hexane as solvents. Response surface methodology (RSM) was employed to optimize parameters influencing oil yield during solvent extraction. A Box Behnken design with three factors: concentration of CaCO3 (1, 1.5, 2%), extraction time (90, 145, 180 min), and extraction temperature (25, 35, 45°C) was utilized for the optimization process. The linear model provided the most accurate fit to the experimental data with determination coefficient (R2) value of 0.8273. The optimized yield of avocado oil was 4.2343% for n-hexane and 6.8156% for ethanol. The fatty acids present in the oil were determined using gas chromatography, and oleic acid was the dominant fatty acid. The values of main physiochemical properties evaluated were: Light Honey brown, Honey brown – colour; Fruity odour, Fruity odour – odour; 150.0114mgKOH/g, 67.32mgKOH/g – saponification value; 1.1809506%, 1.864302% – free fatty acid; 44.892mgI/g, 65.736mgI/g – iodine value; 1.95%, 1.17% – Moisture content; 5.07, 5.04 – pH and 0.291 meq/kg, 0.558 meq/kg – peroxide value for n-hexane and ethanol solvents, respectively. The oil high saponification value and low iodine value makes it applicable in soap making industries, as lubricant in industrial machine and as a stabilizer in other industrial process.
},
year = {2025}
}
TY - JOUR T1 - Extraction of Avocado Seed Oil with Ethanol and N-hexane Using CaCO3 as Coadjuvant: Optimization and Characterization AU - Adeyinka Idowu Alao AU - Zainab Ayotomiwa Lawal AU - Odunayo Deborah Akinwumi Y1 - 2025/09/02 PY - 2025 N1 - https://doi.org/10.11648/j.ajaic.20250802.12 DO - 10.11648/j.ajaic.20250802.12 T2 - American Journal of Applied and Industrial Chemistry JF - American Journal of Applied and Industrial Chemistry JO - American Journal of Applied and Industrial Chemistry SP - 41 EP - 52 PB - Science Publishing Group SN - 2994-7294 UR - https://doi.org/10.11648/j.ajaic.20250802.12 AB - This study aimed to assess the impacts of addition of CaCO3 as a coadjuvant in oil yield from Avocado Pear seed in a soxhlet extraction apparatus with ethanol and n-hexane as solvents. Response surface methodology (RSM) was employed to optimize parameters influencing oil yield during solvent extraction. A Box Behnken design with three factors: concentration of CaCO3 (1, 1.5, 2%), extraction time (90, 145, 180 min), and extraction temperature (25, 35, 45°C) was utilized for the optimization process. The linear model provided the most accurate fit to the experimental data with determination coefficient (R2) value of 0.8273. The optimized yield of avocado oil was 4.2343% for n-hexane and 6.8156% for ethanol. The fatty acids present in the oil were determined using gas chromatography, and oleic acid was the dominant fatty acid. The values of main physiochemical properties evaluated were: Light Honey brown, Honey brown – colour; Fruity odour, Fruity odour – odour; 150.0114mgKOH/g, 67.32mgKOH/g – saponification value; 1.1809506%, 1.864302% – free fatty acid; 44.892mgI/g, 65.736mgI/g – iodine value; 1.95%, 1.17% – Moisture content; 5.07, 5.04 – pH and 0.291 meq/kg, 0.558 meq/kg – peroxide value for n-hexane and ethanol solvents, respectively. The oil high saponification value and low iodine value makes it applicable in soap making industries, as lubricant in industrial machine and as a stabilizer in other industrial process. VL - 8 IS - 2 ER -